April 2025
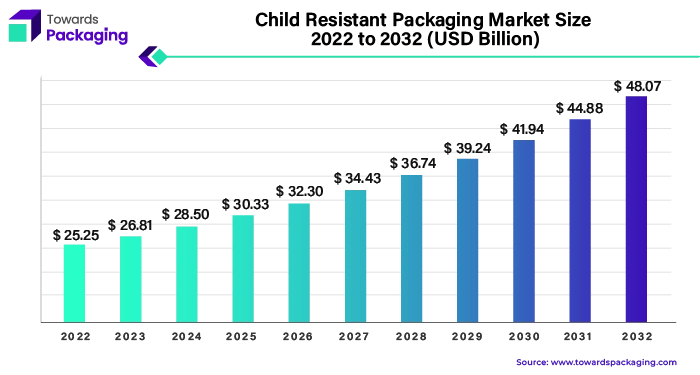
The child resistant packaging market is projected to reach USD 54.71 billion by 2034, growing from USD 30.52 billion in 2025, at a CAGR of 6.7% during the forecast period from 2025 to 2034.
Child-resistant (CR) packaging is made with the express purpose of keeping kids away from the potentially dangerous materials within. The concept is straightforward: children under five should find it difficult to open the packaging, but adults should find it easy. By avoiding unintentional poisonings, this straightforward but efficient method save many deaths every year. Because it plays a vital role in lowering the likelihood that children will access hazardous products, the market for child-resistant packaging, or CR packaging is expanding significantly. CR packaging is made especially difficult for kids to open, yet it still lets most adults see what's inside.
Although there are other options as well, like blister packing and specialty cartons, this is usually accomplished with the use of safety caps and closures. The expanding regulatory requirements that demand the use of CR packaging, especially in industries like pharmaceuticals, are one of the main factors driving this expansion. Prescription pharmaceuticals, over-the-counter remedies, painkillers, and goods containing nicotine, for instance, must frequently be packed in child-resistant packaging. By keeping potentially dangerous materials out of children's reach and securely stowed, this regulatory system lowers the possibility of accidental ingestion.
The importance of CR packaging in preventing infant poisoning incidents cannot be underestimated. Analysis suggests that the use of CR packaging has resulted in a significant decline in the rates of accidental poisoning-related child mortality, with estimates indicating a reduction of up to 45%. Despite these optimistic figures, there are still problems that must be overcome.
One such challenge is the issue of CR package regulations not being followed, especially at the pharmacy point of sale. Noncompliance with these requirements may diminish the efficacy of CR packaging and raise the possibility of youngsters inadvertently consuming it. Medication that was originally prescribed in CR packaging has occasionally been left unattended at home, which has raised the possibility of poisoning occurrences. This highlights the significance it is to have adult supervision and follow safe storage procedures in addition to using CR packaging. Regulations and public awareness of the need to stop paediatric poisoning episodes are driving the expansion of the child-resistant packaging market. While CR packaging has proven to be helpful in lowering death rates, tackling issues such as compliance and good storage techniques is vital for maximising its effectiveness in protecting children's health and wellbeing.
For Instance,
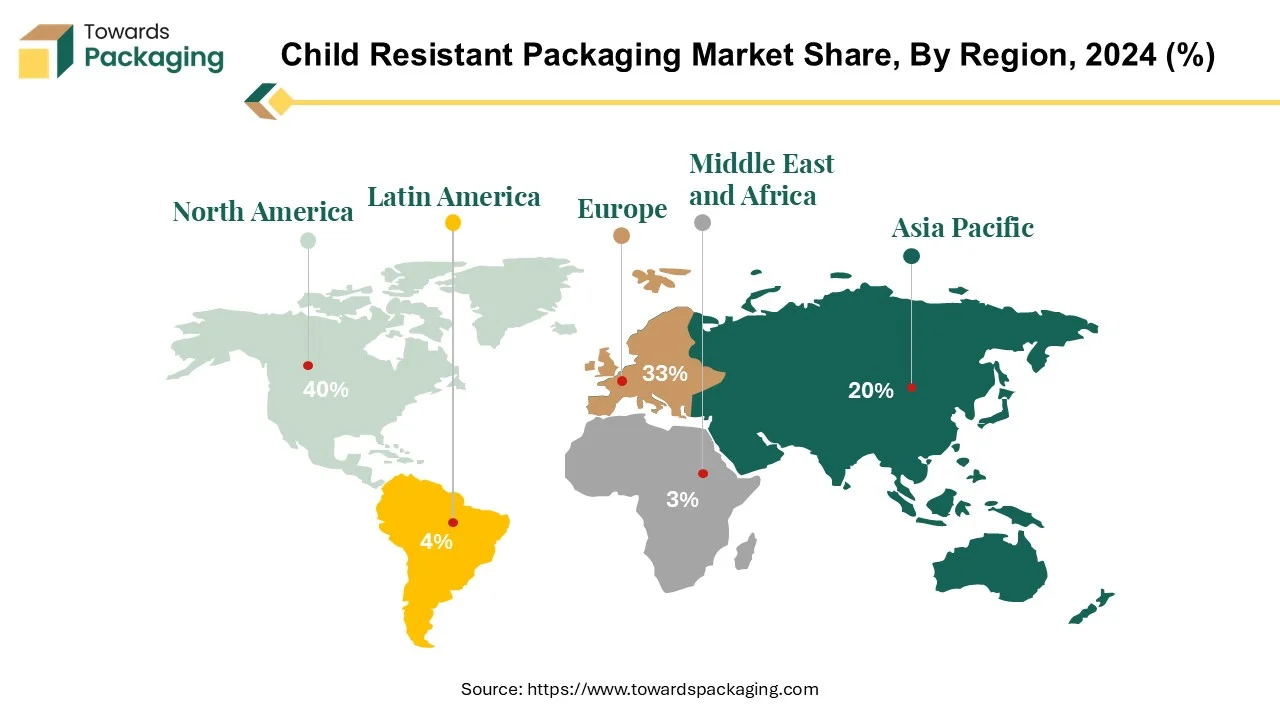
The child-resistant packaging market is dominated by North America due to a multitude of factors, such as increased consumer awareness, technical advancements, and regulatory obligations. The Poison Prevention Packaging Act, which required packaging for potentially hazardous home items including prescription pharmaceuticals and cleaning products to be sufficiently difficult for children under five to open, was responsible for igniting the trend of child-resistant packaging in the United States.
The region's strict product safety laws, especially those upheld by organisations like the Food and Drug Administration (FDA) and the U.S. Consumer Product Safety Commission (CPSC), have been a major factor in the widespread adoption of CRP in a variety of industries, particularly the pharmaceutical industry. According to these requirements, CRP must adhere to certain standards, like being challenging for kids to open while still being usable by senior citizens. Following the legalisation of cannabis products in several states, there has been an increase in child ingesting incidents. Cannabis packaging needs to be child-resistant according to regulations in the US and Canada, and compliance with these regulations is rigorously monitored.
| Child Resistant Import Data, USA, 2024 | |||||
| Date | HS Code | Product Details | Weight In Kg | QTY/UNIT | Country |
| 23-Feb-24 | 710410 | CHILD-RESISTANT UTILITY LIGHTER WITH UN NO. IMO CLASS 1057 2.1 | 19460 | 5420 | China |
| 23-Feb-24 | 290512 | CHILD RESISTANT MULTI-PURPOSE LIGHTER CLASS 2.1 UN 1057 | 16520 | 3450 | China |
| 23-Feb-24 | 290512 | CHILD-RESISTANT UTILITY LIGHTER U | 13668 | 1775 | China |
| 21-Feb-24 | 854221 | 0 CARTONS OF PO: PROD-001189-109346 10 179 20 DRAM GLASS | 57093 | 14400 | Taiwan |
| 19-Feb-24 | 290512 | CHILD RESISTANT LIGHTER, SOLAR LITE US DOT LAA02-00113 | 16275 | 1050 | China |
| 15-Feb-24 | 854221 | 0 CARTONS OF PO: PROD-001189-109343 1 | 57093 | 14400 | Taiwan |
| 14-Feb-24 | 290512 | CHILD RESISTANT LIGHTER, SOLAR LITE US DOT L | 16275 | 1050 | China |
| 14-Feb-24 | 290512 | CHILD RESISTANT UTILITY LIGHTER UN NO. IMO CLASS 1057 2.1 | 15745 | 2892 | China |
| 14-Feb-24 | 940120 | CHILD RESISTANT UTILITY LIGHTER IMO HAZARD CLASS 2.1 UN | 17400 | 8700 | China |
| 14-Feb-24 | 961320 | CHILD-RESISTANT POCKET LIGHTER, GAS REFILLABLE CLASS:2.1 | 19923 | 1070 | China |
In the US, child-resistant import data is usually collected via a variety of sources, such as trade and customs officials. When shipping products with child-resistant packaging, importers must give comprehensive details about the shipment, such as the kind of packaging, quantity, value, and country of origin. The packaging materials and design sector in North America has benefited greatly from technical improvements. Businesses are spending money on R&D to produce innovative CRP solutions that not only improve safety but also satisfy changing consumer preferences. Examples of these solutions include child-resistant closures, blister packs, and customised cartons.
North America's position as a major participant in the worldwide CRP market is cemented by its aggressive regulatory framework, continuous technical innovation, and increased consumer awareness. It is anticipated that North America will continue to be at the forefront of CRP development and adoption as long as there is a sustained focus on children protection and product safety.
For Instance,
Europe is a major player in the child-resistant packaging (CRP) industry, which is growing at an impressive pace due to a variety of issues. Comparable to those in North America, regulations play a key role in determining the acceptance of CRP across Europe. The European Union (EU) has implemented rules and regulations with the objective of enhancing product safety and protecting children from unintentional consumption of hazardous materials. Demand for CRP has surged in Europe because to growing consumer and carer awareness of child safety. Growing increasingly aware of the concerns connected to home products and pharmaceuticals, parents actively look for products with CRP to lessen these risks.
A non-reclosable child-resistant pack is defined by the European standard BS EN 14375:2003 as one that cannot be properly sealed again after it has been opened and part or all of its contents removed. Blister and strip packs are common examples, as they are used for medications and single-use household goods. A polymer tray that has been sealed with aluminium foil usually makes up blister packs. Creating a two-step opening procedure, such as "peel and push," or using a paper label that prevents children' attempts to open it with their little fingernails are common ways to incorporate child resistance.
In the UK and the EU, child-resistant packaging has developed into an established valuable product that complies with strict safety standards and laws. Due to governmental regulations, increased consumer awareness, and technical advancements, the CRP industry in Europe is expanding gradually. The demand for CRP is expected to increase in the region in the future as product safety and children protection are prioritised. The European CRP market is expected to grow further as new safety regulations and regulatory frameworks are established.
For Instance,
Child safety, particularly the prevention of poisoning, is a crucial aspect of medicine packaging. In the early 1970s, child-resistant packaging was introduced in the UK. By 2008, the World Health Organization (WHO) hailed it as the “best documented cause of the reduction of child poisoning in the developed world.”
Since its inception, child-resistant packaging has evolved significantly and is now widely accepted in the UK, EU, USA, Canada, and Australia. It is also rapidly gaining acceptance in China, other Southeast Asian countries, and India. It's important to note that child-resistant packaging is not "child proof"; rather, it is designed to be difficult for children younger than 52 months to open, while remaining accessible for adults. This definition is outlined in ISO 8317:2015, one of the three ISO standards governing this type of packaging outside the USA. In the United States, the regulation 16 CFR 1700.20 provides a similar definition of “special packaging.”
ISO 8317:2015 is a testing standard for re-closable, child-resistant packaging for any content. The other two international standards are ISO 14375:2018, which covers non-reclosable packaging for medicines, and ISO 28862:2018, which addresses non-reclosable packaging for non-pharmaceutical products. Additionally, ISO 13127:2012 outlines mechanical test methods for packaging that has been tested for compliance with ISO 8317 but has undergone minor changes.
Through these standards and continuous development, child-resistant packaging has become an essential tool in protecting children from accidental poisoning while ensuring ease of use for adults.
Plastics and materials with a high plastic content play a major role in child-resistant packaging (CRP), which is essential to product safety. While blister packs frequently combine paper and aluminium foil and reclosable packs may include glass containers, plastics like PP, HDPE, LDPE, or PVC continue to be the primary components used in the production of CRP. This dependence highlights the plastics industry's significance in CRP production.
Plastic push-turn tops are a popular way among many used to create child-resistant packaging, specifically for full glass or plastic containers. The contents of CRP must not have an impact on its performance. For instance, particular pharmaceutical ingredients in liquid formulations may not combine properly with certain types of plastic, which could reduce the CRP's efficacy.
Growth in the plastics industry is being driven by the increasing demand for CRP. The demand for an efficient CRP grows as laws become more stringent and consumers' knowledge of product safety rises. The development of innovative CRP solutions that satisfy strict safety requirements and guarantee consumer convenience is made possible by improvements in plastics technology. To satisfy the changing needs of the child-resistant packaging market, the plastics industry has become essential.
For Instance,
Child-resistant caps and closures have been recognised by authorities such as the World Health Organisation as extremely useful instruments in averting unintentional poisoning incidents in children, thereby decreasing medication-associated fatalities globally. While bottles and containers can be designed child-resistant by a variety of elements, the cap is the main part that makes sure the packaging is child-resistant.

The leading importers of child-resistant caps are the US, Pakistan, and India, who together account for a sizable portion of the market for child-resistant packaging. Demand is driven by India's burgeoning pharmaceutical sector and manufacturing prowess. The US encourages innovation and compliance because of its strict safety rules. Demand is fueled by Pakistan's developing pharmaceutical sector, which reflects the expansion of the regional market and the adoption of child-resistant packaging requirements.
These caps are available in various styles that are specifically made to work with materials like glass or plastic bottles. For example, plastic bottles—especially the amber ones used in pharmacies for the storage or administration of medications—often have push-turn threaded caps manufactured of polypropylene (PP). Plastic push-turn caps are adaptable and widely used in the production of child-proof package combinations with sturdy glass or plastic containers. It's essential to ensure that the chosen caps and bottles meet regulatory standards set by bodies like the Consumer Product Safety Commission (CPSC) to guarantee their effectiveness in child-resistant packaging.
Manufacturers provide comprehensive packages for child-resistant packaging, including push-turn dome-capped PP plastic dropper bottles, which simplify the packing process and guarantee regulatory compliance.
Child-resistant plastic caps provide more than just protection prevent accidental consumption; these have several additional beneficial functions. They are affordable, recyclable, and work with several kinds of bottles, such as jugs and vials. For packaging tinctures and liquid medications in glass and plastic bottles, for example, child-resistant capable dropper caps are perfect since they offer both convenient application and child protection.
Child-resistant closures continue to be developed. One such development is the SecureCapsTM two-piece push and turn closure, which satisfies strict certification requirements for childproof packaging and works with pharmaceutical bottles that have standard neck sizes of 28 mm or less. These advancements highlight the industry-wide commitment to improving child safety in packaging even more.
For Instance,
The pharmaceutical industry makes widespread use of child-resistant packaging (CRP) to reduce the possibility of accidental consumption of hazardous materials, especially by young patients. To guarantee the safe storage and dispensing of pharmaceutical medicines, regulatory organisations such as the Food and Drug Administration (FDA) and the U.S. Consumer Product Safety Commission (CPSC) require the use of CRP.
Pharmaceutical packaging meets several needs beyond basic package specifications. Two of its most important roles are accurately conveying vital information and protecting the purity of pharmaceuticals. Pharmaceutical packaging, in contrast to typical consumer packaging, is governed by strict regulations since mistakes could have fatal implications. Designing pharmaceutical packaging with child-resistant features and geriatric user accessibility considerations is essential.
Despite advances in pharmaceutical packaging, difficulties remain, requiring greater care to avoid life-threatening instances. Every year, inadvertent medicine poisoning results in over 59,000 children in the United States needing emergency room care, according to Safe Kids Global.
The significant reduction in child poisoning occurrences resulting from inadvertent exposure to medications is indicative of the effectiveness of CRP. Research indicates that the introduction of CRP has resulted in a significant decrease in medication-associated deaths and ER visits caused by unintentional overconsumption. CRP regulations maintain strict guidelines for testing and packaging design to guarantee the effectiveness of child-resistant systems. CRP remains an essential element of pharmaceutical packaging strategy, supporting industry efforts to put patient safety first and avoid medication-related accidents.
The competitive landscape of the child resistant packaging market is dominated by established industry giants such as Amcor plc (Switzerland), Bilcare Limited, WestRock Company (U.S.), Gerresheimer AG (Germany), Closure Systems International (CSI), Constantia Flexibles (Austria), Winpak Ltd (Canada), Bemis Manufacturing Company (U.S.), KushCo Holdings, Berry Global Group, Inc. (U.S.), O.Berk Company, LLC (U.S.), Sun Grown Packaging (U.S.), MJS Packaging (U.S.), Kaufman Container (U.S.), Ecobliss B.V. (Netherlands), Global Closure System (France), Syntegon Technology GmbH (U.S.), ABC Packaging Direct (U.S.), Carow Packaging Inc (U.S.), Colbert Packaging (U.S.), Comar (U.S.), LA Packaging (U.S.), Mold-Rite Plastics (U.S.), Origin Pharma Packaging (U.K.), Parkway Plastics Inc (U.S.). These giants compete with upstart direct-to-consumer firms that use digital platforms to gain market share. Key competitive characteristics include product innovation, sustainable practices, and the ability to respond to changing consumer tastes.
The Swiss company Amcor plc strategically concentrates on sustainability and innovation in the child-resistant packaging (CRP) industry. Amcor wants to create innovative CRP solutions that satisfy strict safety regulations and take environmental considerations into account. To this end, the company is making use of its global footprint and significant research and development resources. To ensure efficacy and user-friendliness, the company places a strong emphasis on collaborating with pharmaceutical companies to customise CRP solutions to particular medicine requirements.
For Instance,
A major participant in the CRP industry, Bilcare Limited, takes a customer-centric stance by providing pharmaceutical companies with tailored CRP solutions. In order to guarantee that CRP satisfies customer demands and regulatory standards, the firm highlights the significance of cooperation and consultation throughout the design and development process.
For Instance,
The US-based WestRock Company is dedicated to offering complete packaging solutions, such as CRP, to a variety of sectors, including the pharmaceutical sector. The firm creates innovative CRP solutions that put user convenience and kid safety first by utilising its vast experience in packaging design and manufacture.
For Instance,
German-based Gerresheimer AG is an expert in pharmaceutical packaging solutions, such as CRP, that are designed to protect the integrity and safety of drugs. The company adheres to stringent standards throughout the manufacturing process to ensure the efficacy of its CRP products, with a significant emphasis on regulatory compliance and quality assurance.
For Instance,
By Material
By Packaging Type
By End User
By Region
April 2025
April 2025
April 2025
April 2025